Problem: How can we use water displacement to calculate the volume of one penny?
Materials:
- Volume of a Penny Lab (PDF)
- Graduated cylinders (25 mL, 50 mL, or 100 mL)
- Cup or beaker of water, food coloring optional
- Pennies – 100+ per group
- Tub
- Plastic Spoon – to pour water out of graduated cylinder and separate pennies

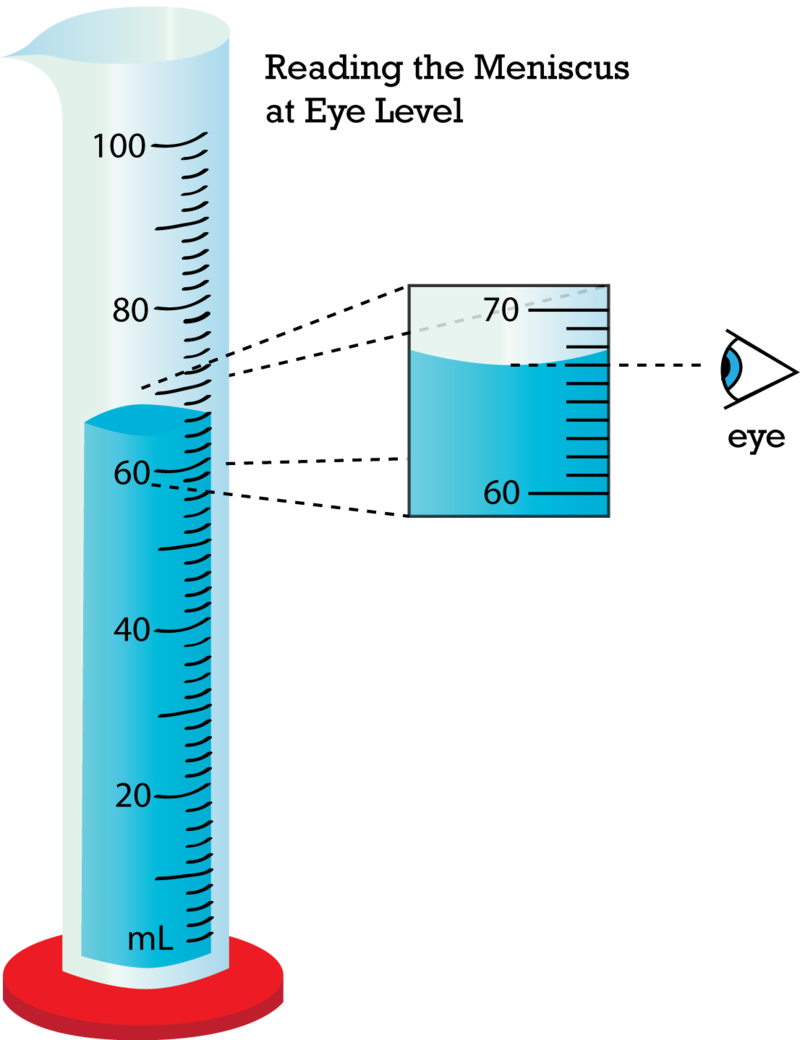
This is a simple & fun lab to have students practice measuring and reading volume as well as use water displacement to determine the volume of a penny – an irregularly shaped object.
Students will design their own series of 10 tests with the following criteria:
- All pennies must be under water inside of the graduated cylinder.
- The volume of water must not pass the 100 mL (or highest) increment.
- All data is recorded carefully.
Students were able to carefully measure and determine that the volume of a penny was 0.35 mL – most students were very close with a range of 0.33 – 0.37 mL.